Solved in the t−v diagram shown below, process 1−2 is a: 2.3 phase diagrams – introduction to engineering thermodynamics Mech_feed_shop: slope of constant volume and constant pressure
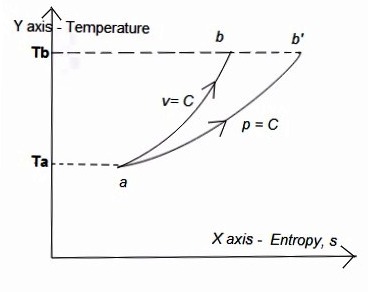
Representation of constant volume and constant pressure process on TS
How to draw a pv diagram
Isochoric process
What is reversible of constant volume processIsochoric process – formula, work done, and diagram Constant volume process (isochoric process)Volume constant diagram process pt.
Volume constant process reversible thermodynamics[diagram] pv diagram constant Constant volume processes in thermodynamicsThermodynamics chapter 2.

Ts diagram constant pressure
Volume constant process chemical thermodynamics advance engineering ppt powerpoint presentation work slideserveSolved consider a process that occurs at constant volume. Constant volume work process energy transfer mass heat ppt powerpoint presentationConstant diagram volume ts pressure process.
Solved show for constant volume process between state 1 andP-v and t-s diagrams Constant mechIsothermal process.

Mech_feed_shop: slope of constant volume and constant pressure
Temperature-entropy(t-s) diagramConstant volume process Constant-volume process on pt diagram (interactive)Constant volume process.
Thermodynamic processes: isobaric, isochoric, isothermal and adiabaticConstant volume process temperature diagram eme 2nd chapter notes ii Schematic diagram showing the pt path of a constant volume, constantDiagram adiabatic process constant pressure diagrams processes represent following eme 2nd chapter notes.

Representation of constant volume and constant pressure process on ts
Ctiemt cse 2012-2016: eme notes (2nd chapter)[diagram] internal combustion engine pressure volume diagram Volume constant processes thermodynamics chemistry here qsstudyThermodynamics chapter volume constant.
Ctiemt cse 2012-2016: eme notes (2nd chapter)Isometric process Constant volume processes in thermodynamicsObjectives_template.

Operator generic fundamentals – thermodynamic cycles
[diagram] t s diagram .
.




![[DIAGRAM] Pv Diagram Constant - MYDIAGRAM.ONLINE](https://i2.wp.com/revise.im/content/02-physics/05-unit-5C/02-first-thermodynamics/constant_volume_pressure.png)

